NAFDAC ALERTS PUBLIC ON SUSPECTED FALSIFIED AUGMENTIN 625MG
By Adeola Abdullah
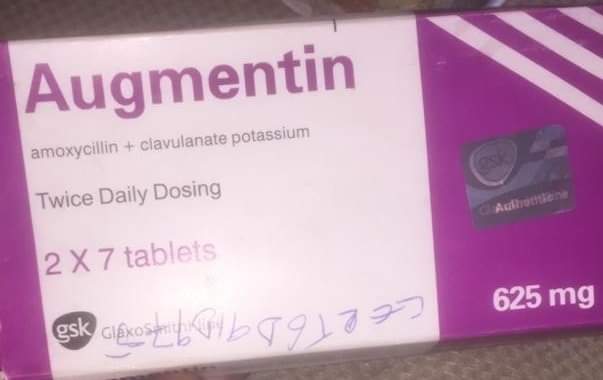
The National Agency for Food and Drug Administration and Control (NAFDAC) has drawn the attention of the public to the detection of suspected falsified Augmentin 625mg Tablets in circulation within the country with the following details and labelling lapses;
▪︎Product Name: Augmentin 625mg.
▪︎Batch No.: 562626
▪︎Manufacturing date: April 2021
▪︎Expiry date: April 2024.
▪︎NAFDAC Reg No: 04-1928.
Labelling Lapses;
➡️ The product failed short of the labeling requirements.
➡️ No inscription “manufactured by” is written on the label -only the address.
➡️ Manufacturing and Expiry dates do not meet the acceptable format.
➡️ No MAS scratch number for verification.
➡️ The logo “gsk” is not properly positioned as on the original.
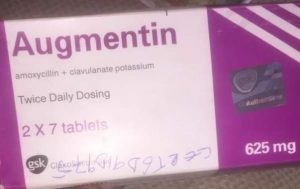


The listed information indicates the product is falsified and counterfeited.
In view of the above, NAFDAC has notified all its formations in the zones and 36 states of the federation including the FCT to carry out surveillance and mop up the falsified Augmentin tablets.
The release further stated that the genuine Augmentin 625mg has legible product labelling information including date markings – expiration and manufactured dates, batch number and NAFDAC registration number.
“Members of the public in possession of the above suspected counterfeit product are advised to discontinue sale or use and submit stock to the nearest NAFDAC office.
Report any suspicion of adverse drug reaction and substandard and falsified medicines to NAFDAC on 0800-162-3322 or sf.alert@nafdac.gov.ng”
More Stories
WHITNEY ADENIRAN: THIRD DEFENDANT’S COUNSEL DISAGREE WITH WITNESS ON WHO IS RESPONSIBLE FOR SAFETY PRECAUTIONS AT THE STADIUM
At the resumption of the cross-examination of the third prosecuting witness in the trial of Whitney Adeniran, Olukayode Enitan, SAN,...
CRIME: MY 12-YEAR-OLD DAUGHTER WAS DEFILED BY A FAMILY FRIEND – FATHER TELLS COURT
By Aishat Momoh. O. A witness (name withheld) has described to an Ikeja Sexual Offences and Domestic Violence Court how...
BREAKING: EFCC DECLARES EX-KOGI GOV, YAHAYA BELLO WANTED
The Economic and Financial Crimes Commission has declared a former governor of Kogi State, Yahaya Bello, wanted for offences relating...
I’M STILL IN CHARGE, GANDUJE BOASTS AS COURT HALTS SUSPENSION
Former governor of Kano State, Senator Abdullahi Ganduje, said he is still in charge as the national chairman of the...
PDP BoT QUERRIES CONTINUED STAY OF DAMAGUM, ANYANWU
The Peoples Democratic Party (PDP) Board of Trustees (BoT) has said the controversy surrounding the leadership of the party at...
STUDENTS LOAN: 1.2M BENEFICIARIES TO BE IN FIRST BATCH
The Nigerian Education Loan Fund (NELFUND) says the Unified Tertiary Matriculation Examination registration number, National Identification Number and Bank Verification...